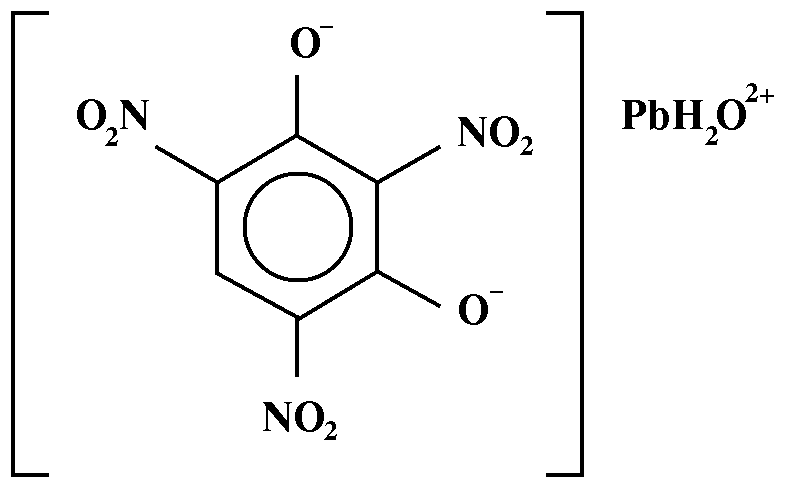
- Chemical Name: lead 2,4,6-trinitroresorcinate
- Chemical Formula: C6H3N3O8Pb
See also: Kirk-Othmer Encyclopedia of Chemical Technology, John
Wiley & Sons, Inc., New York, 1993, Volume 10, Explosives and
Propellants, Pages 5, 20-21.
- CAS Registry Number: [15245-44-0]
- Detonation Temperature: 260°C (500°F)
Hawley's Condensed Chemical
Dictionary, 13th Edition, Revised by Richard J. Lewis, Sr.,
John Wiley & Sons, Inc., New York, 1997, Page 665.
- Volume of lead styphnate in each 700 μm diameter by 1 mm deep
combustion chamber: 0.120 mm3.
- Density of lead styphnate: 1.5 mg/mm3.
- Mass of lead styphnate in each combustion chamber: 180 μgram.
- There are two forms of lead
styphnate: six-sided monohydrate crystals and small rectangular crystals.
Lead styphnate varies in color from yellow to brown. Lead styphnate is
particularly sensitive to fire and the discharge of static electricity. When
dry, it can be readily detonated by static discharges from the human body. The
longer and narrower the crystals, the more susceptible lead styphnate is to
static electricity. Lead styphnate does not react with metals and is less
sensitive to shock and friction than mercury fulminate or lead azide. Lead styphnate is
only slightly soluble in water and methyl alcohol and may be neutralized by a
sodium carbonate solution. It is used as a component in primer and detonator
mixtures. It is stable in storage, even at elevated temperatures.
Quoted from: http://www.ordnance.org/
- 13-1. Properties of Initiating Explosives. Initiating explosives include
lead azide, mercury fulminate, lead styphnate and tetracene. They are very
sensitive to friction, heat, and impact. When involved in a fire, they can be
expected to detonate without burning. Quantities in storage and in process
must be limited to the smallest practicable amounts. Bulk initiating
explosives will be stored in conductive containers and if more than 10 grams
are stored for more than 4 hours, they shall be kept wet with water or with
water-alcohol mixtures. Every effort shall be made to prevent the liquid from
freezing, and if frozen, explosives material itself shall be handled. Whenever
processing requires the scooping or pouring of dry initiating explosives, the
operation will be done by remote control. Dust from initiating explosives
operations shall be collected with a wet-type aspirator system. The aspirator
bottle or container shall be located as close to the dust intake point as
practicable. The aspirator bottle will contain an approved desensitizing agent
or be housed in a protective shield. No valves, where explosives may lodge,
shall be in the vacuum line. The vacuum will be controlled to preclude
excessive bubbling. Because explosives may be present, extreme caution will be
used when disassembling the system to clean it. Contaminated sections of
vacuum systems shall be cleaned daily by circulating an approved desensitizing
solution through the tube or pipe. Dry-type collection systems will not be
used. Emphasis must be placed upon cleanliness and general housekeeping since
contamination of these explosives with foreign or gritty material markedly
increases their sensitivity. Rooms in which initiating explosives are handled
shall have floors of lead or other nonsparking flooring material. Flooring
shall always be of conductive finish. Walls of the rooms should be covered
with waterproof material having a smooth hard gloss finish. Frequent washing
of the rooms with a neutralizing solution is necessary. Drying of the
explosives is usually accomplished in muslin squares on a drying table or by a
special air blowing device with temperatures limited to between 122 degrees
Fahrenheit and 140 degrees Fahrenheit (50 degrees Celsius and 60 degrees
Celsius). Bulk initiating explosives shall be packaged and transported in
accordance with current DOT regulations pertaining to the specific initiating
explosive.
b. Lead styphnate. This explosive is particularly sensitive to discharge of
static electricity, and the dry material can be readily ignited by static
discharges from the human body. Lead styphnate is approximately as sensitive
as mercury fulminate to impact and has about the same order of friction
sensitivity as lead azide. It should be stored under water in conductive
rubber containers. Where practicable, lead styphnate should be in the
water-wet state while being processed. Water should be removed by decanting.
It is usually dried by suction filtering, washing with alcohol, and drying in
an oven at 50 to 60 degrees Celsius. The alcohol wash is needed to prevent
caking since breaking up caked explosives is hazardous. Conventional methods
of de- watering explosives such as placing material in cloth then squeezing
and draining on inclined smooth surfaces such as glass are not recommended. To
remove styphnate from receptacles, a stream of water should be used to wash
the material from the inclined container. If this procedure is impractical,
the styphnate may be carefully removed by hand, provided rubber gloves are
worn. The use of spatulas, rakes, or scoops should be prohibited. Containers
equipped with removal rubber liners facilitate handling of the wet explosives
and are recommended. Lead styphnate tends to form a sensitive scaly deposit on
the sides of the containers and collection sumps. The scale can be removed
with 5 to 10 percent sodium hydroxide or sodium acetate solutions. The removal
of the scale with tools or other instruments shall not be attempted.
Operations should provide for eye protection. Conductive flooring and table
tops, without cracks or crevices in which explosives can lodge, are required.
Conductive footwear is required. All equipment shall be electrically grounded.
Quoted from: http://www.redstone.army.mil/safety/reg385100/385ch13.html
- 1973-10032
18 Sep 73
24P
FRANKFORD ARSENAL PHILADELPHIA
PA
SUSCEPTIBILITY OF ELECTRIC PRIMERS TO ELECTROSTATIC
DISCHARGE.
Meeting Paper
Schlack, A. F.
This paper was presented at
the Fifteenth Explosives Safety Seminar, held at the Hyatt Regency Hotel, San
Francisco, CA on 18-20 September, 1973, Vol. I, AD 775-580 (73-10001), P
637-660.
Availability: NTIS/DTIC - Approved for public release;
distribution is unlimited.
Priming mixtures made with lead styphnate are highly sensitive to
electrostatic discharge. If stray static charges ignite the primer during
handling, serious injury and/or damage could result, especially if the primer
is assembled in a loaded cartridge. Therefore, several studies were initiated
at Frankford Arsenal to decrease the susceptibility of the primer to
electrostatic discharge by modifying the priming composition. It was found
that the addition of boron to the composition decreased its electrostatic
sensitivity. Another study indicated that substituting Trisal for lead
styphnate also decreased the mixture's sensitivity. Plans for future studies
are also discussed in the paper.
SEARCH TERMS: 1: ELECTROSTATIC HAZARDS 3: priming mixture 4: lead styphnate
5: electrostatic sensitivity 1: ELECTROSTATIC HAZARDS 3: priming mixture 4:
lead styphnate 5: experimental study 1: HANDLING HAZARDS 3: electric primer 4:
electrostatic sensitivity 5: lead styphnate 1: PERSONNEL HAZARDS 3:
electrostatic initiation 4: electric primer 5: lead styphnate 1: INITIATORS 3:
lead styphnate 4: electrostatic sensitivity 5: experimental study 6: boron 6:
voltage sensitivity test 6: M52A3B1 primer 6: 20mm ammunition
Quoted from: Johns Hopkins University, Chemical Propulsion Information
Agency: http://www.jhu.edu/~cpia/ddesbdat/7310032.html
- Material Safety Data Sheets:
From: Olin Brass and Winchester Ammunition, Inc.: http://www.winchester.com/contactus/winmsds.htm










